| Anyone who has tried to quit the anti-depressant ‘Paxil’ will tell you that the TV ads which claim that Paxil is not ‘habit-forming’ is misleading. Many people who take Paxil experience SEVERE withdrawal symptoms when trying to get off the drug, from tremors to cold sweats to “electric zaps to the brain” (not painful but very uncomfortable). The Paxil withdrawal is SEVERE. What is concerning is that consumers tend to believe that drug ads are regulated. According to one recent study(6), some 43% of participants believed, falsely, that only ‘completely safe’ drugs could be advertised.
As i said at the beginning of this article, drugs are sometimes appropriate and at times can save a person’s life, but most of the time they are unnecessary, expensive and harmful. You would be well advised to recognize that you should seek natural therapies which address the cause of the disease before choosing a drug-based solution.
46% of Americans take at least one prescription drug daily. (1)
Although drugs are sometimes appropriate and at times can save a person’s life, most of the time they are unnecessary, expensive and harmful. My advice would be to recognize that you should seek natural therapies which address the cause of the disease before choosing a drug-based solution.
I can assure you that the number of people who actually need drugs is a small percentage of those taking them. For example, people are being prescribed drugs for heartburn when it is one of the easiest health problems to treat. Most people ignore the fact that heartburn is an important clue from their body and rely on a drug instead to suppress the symptoms.
In case you haven’t figured it out by now, the major reason for the traditional medical paradigm is the influence of the most powerful industry in the United States, the drug companies.
Drug companies exert a major influence on the majority of studies published and nearly all of medical education. This influence is what causes doctors to use their expensive symptomatic ‘patch-up’ drugs as solutions for people’s problems.
Drug companies have been able to get away with their high prices because the vast majority of people do not pay for their medications anymore. It’s insurance companies that are picking up the tab. Since most people do not pay for it directly, drug companies are able to get away with charging outrageous prices.
Drug companies are the most profitable industry
The drug companies claim that they need large earnings – 125,835,595,000 in 1999 (2) – to conduct their research and development. They have a point – only up to a degree. Aggressive research is indeed needed. The medications produced by the pharmaceutical industry have improved the quality and length of life of many people. But this justification loses credibility when:
- Just 1 out of every 5 dollars the drug industry collects goes to drug research.
- Some drug companies spend almost twice as much money for advertising and marketing as they spend for research.
- Drug industry profits are so large they outstrip every other industry’s profits by far (3).
Drug companies are the most profitable industry. In 2001, a year which saw a drop in employment rates, a plunge in the stock market and symbols of America’s economy literally come crashing down, the drug companies continued their reign as the most profitable industry in the annual Fortune 500 list.
While the overall profits of Fortune 500 companies declined by 53%, which was the 2nd biggest dive in profits the Fortune 500 has taken in its 47 years, the top 10 U.S.
 Collectively, the 10 drug companies in the Fortune 500 topped all 3 of the Fortune magazine’s measures of company profitability for 2001, according to the magazine’s annual analysis of America’s most important companies. Collectively, the 10 drug companies in the Fortune 500 topped all 3 of the Fortune magazine’s measures of company profitability for 2001, according to the magazine’s annual analysis of America’s most important companies.
These drug companies had the greatest return on revenues, reporting a profit of 18.5 cents for every $1 of sales, which was 8 times higher than the median for all Fortune 500 industries, easily surpassing the next most profitable industry, which was commercial banking with a 13.5% return on revenue)(3).
The system is badly broken and in need of a change. We cannot spend over one trillion dollars for health care just to improve profits for drug companies. We have the capital to more than adequately treat nearly all people. What we need to do is shift our perspectives and priorities.
This emphasis on drugs is one of the main reasons why spending for prescription drugs is the fastest-growing category of health care expenditures.
It is also one of the major factors contributing to the fact that doctors are a major leading cause of death in the United States, due to the fact that they have an over reliance on using drugs as ‘patch-up’ solutions, rather than seeking the cause of the problem.
How safe are prescription drugs?
In 1998 an extensive study published in the reputable Journal of the American Medical Association (JAMA) showed that 106,000 people die each year in American hospitals from medication side effects (4).
Let’s look at this statistic a different way: 106,000 deaths a year averages out to nearly 300 deaths per day, every day. Deaths from all major airline crashes in the U.S. average less than 300 annually, but 1 airplane crash gets more media attention and governmental scrutiny than the 300 medication-related deaths which occurred not only on the same day as the airline crash, but also every day before and after for decades.
Why has this epidemic of side effects gone unrecognized? Deaths from medication reactions rarely look any different from natural deaths. There’s no visible wreckage to videotape, no crash sites to fascinate and horrify TV viewers. As media people say, ‘No film, no story’. Media and public relations firms, and how they shape the public’s awereness, are discussed in more detail here.
Medication deaths often occur quietly in hospitals, emergency rooms and private homes. When medication-related deaths occur, it’s often unclear at first whether the cause was the medication, the illness, or some other factor. In other words, to much of the media, there is nothing sexy about side effects.
The reported adverse effects of drugs are only the tip of the iceberg. Consider ‘Digoxin’, the best-selling heart drug. According to an article in JAMA, the Food and Drug Administration (FDA) receives about 82 reports each year involving Digoxin, yet a systematic study of Medicare records reveals 202,211 hospitalizations for Digoxin adverse effects in a 7-year period (5). That’s more than 28,000 reactions per year, 82 of which the Food and Drug Administration (FDA) hears about.
Think you know for sure that the drug you are taking is absolutely safe? Think again. Many drugs spend years on the market before being taken off the market because of dangerous side-effects which surface. Aggressive marketing, slanting research, unethical publishing of results, influencing physicians, intimidating researchers, pressuring medical centers, manipulating the FDA, limiting information, marketing drugs with inaccurate safety information – all of these have created an environment in which drug development has become a race for the bottom line.
Knowing how the drug companies operate, it is no surprise when new dangers are revealed with drugs we’ve been using for decades and drugs are subsequently taken off the market.
 Some of these withdrawn drugs, such as ‘Redux’, ‘Seldane’, ‘Propulsid’, ‘Rezulin’ , were prescribed MILLIONS OF TIMES. According to Dr. Alastair J.J. Wood, Assistant Vice Chancellor for Research at the Vanderbilt University Medical Center (1), a staggering 19.8 million patients (almost 10% of the United States population) were estimated to have been exposed to just 5 of the 10 drugs withdrawn in the past 10 years. (see below for the list) Some of these withdrawn drugs, such as ‘Redux’, ‘Seldane’, ‘Propulsid’, ‘Rezulin’ , were prescribed MILLIONS OF TIMES. According to Dr. Alastair J.J. Wood, Assistant Vice Chancellor for Research at the Vanderbilt University Medical Center (1), a staggering 19.8 million patients (almost 10% of the United States population) were estimated to have been exposed to just 5 of the 10 drugs withdrawn in the past 10 years. (see below for the list)
Dr. Wood added, “None of the drugs was indicated for a life-threatening condition nor, in many cases, were they the only drugs available for that indication”.(2) Safer alternatives to these drugs existed, but intense marketing convinced doctors to prescribe them anyway.
Drug companies can profit handsomely from such drugs. ‘Seldane’, the top-selling antihistamine in the world for more than a decade, was on the market for 13 years until the Food and Drug Administration (FDA) removed it in 1997, 7 years after the drug’s cardiac toxicities were identified in 1990 (3).
Rezulin’, a diabetes drug withdrawn by Britain in 1997, wasn’t withdrawn by the Food and Drug Administration (FDA) until 2000, during which time Warner-Lambert earned $1.8 billion (4).
10 prescription drugs withdrawn from the market since 1997
These drugs were taken off the market because of serious, often lethal side effects.
| Rezulin: Given fast-track approval by the Food and Drug Administration (FDA), Rezulin was linked to 63 confirmed deaths and probably hundreds more. “We have real trouble,” a Food and Drug Administration (FDA) physician wrote in 1997, just a few months after Rezulin’s approval. The drug wasn’t taken off the market until 2000.
Lotronex: Against concerns of one of its own officers, the Food and Drug Administration (FDA) approved Lotronex in February 2000. By the time it was withdrawn 9 months later, the Food and Drug Administration (FDA) had received reports of 93 hospitalizations, multiple emergency bowel surgeries, and 5 deaths.
Propulsid: A top-selling drug for many years, this drug was linked to hundreds of cases of heart arrhythmias and over 100 deaths.
Redux: Taken by millions of people for weight loss after its approval in April 1996, Redux was soon linked to heart valve damage and a disabling, often lethal pulmonary disorder. Taken off the market in September 1997.
Pondimin: A component of Fen-Phen, the diet fad drug. Approved in 1973, Pondimin’s link to heart valve damage and a lethal pulmonary disorder wasn’t recognized until shortly before its withdrawal in 1997.
Duract: This painkiller was taken off the market when it was linked to severe, sometimes fatal liver failure.
Seldane: America’s and the world’s top-selling antihistamine for a decade, it took the Food and Drug Administration (FDA) 5 years to recognize that Seldane was causing cardiac arrhythmias, blackouts, hospitalizations, and deaths, and another 8 years to take it off the market.
Hismanal: Approved in 1988 and soon known to cause cardiac arrhythmias, the drug was finally taken off the market in 1999.
Posicor: Used to treat hypertension, the drug was linked to life-threatening drug interactions and more than 100 deaths.
Raxar: Linked to cardiac toxicities and deaths. |
Drug companies are a major influence on doctors’ prescribing habits
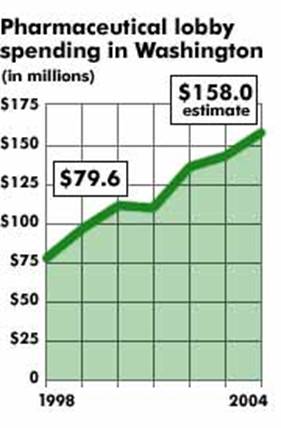
In addition to the 3 billion dollars they spend on direct marketing to consumers, drug companies are spending about 15 billion dollars per year on marketing to doctors.
Most physicians have no idea that the drug companies are spending on average $10,000 per doctor to influence their behavior. The doctors do not receive a check, of course, but the perks are significant.
Doctors also don’t realize that they actually lose that much income and more if they factor in the time that they lose by sitting with the drug company representatives and going to their ‘free’ lectures and meals. Doctors also often overlook what a fiduciary responsibility is, and therefore don’t realize that they need to analyze carefully the costs involved in recommending expensive prescription drugs.
Doctors cause patients to divert much of their hard-earned income to the drug companies, which further perpetuates this indirect physician subsidy. All this results in prescription drugs being the fastest-growing category of health care expenditures in the United States.
MEDICATIONSENSE.COM
By Jay S. Cohen M.D.
“Informed consent can be effectively exercise only if the patient possesses enough information to enable an intelligent choice” (AMA, 1999).
THE MEDICAL PROFESSION’S CULTURE OF CORRUPTION, PART 1: Respected Physicians Call for End of Conflicts of Interest with Drug Industry.
There have been many calls for reform of the broad influence of pharmaceutical industry money on doctors and other health professionals, hospitals, and medical centers, but few have been so sweeping as the recent article in the Journal of the American Medical Association by Dr. Troyen Brennen of Harvard Medical school, Dr. Jerome Kassirer, who was an editor of the New England Journal of Medicine, and 9 other authors.1
The drug industry has been roundly criticized for its intense, diverse, and unrelenting efforts to influence doctors and sell more drugs. The criticism has accomplished little, and drug sales have soared. The analysis by Brennen et al. focused on the medical profession, acknowledging that “physicians’ behavior is a large part of the problem,” and that the stature of the medical profession and the trust of patients have been jeopardized by medicine’s many conflicts of interest with the drug industry.
Approximately $19 billion is spent annually by drug companies for marketing to doctors. Tens of thousands of sales representatives descend on doctors’ offices every day. Patients in doctors’ waiting rooms are often outnumbered by drug reps (typically young, female, attractive). Many doctors deny that gifts and other freebies influence their decisions about medication treatment. Drs. Brennen et al. disagreed:
“Social science research demonstrates that the impulse to reciprocate for even small gifts is a powerful influence on people’s behavior. Individuals receiving gifts are often unable to remain objective …. Receiving gifts is associated with positive physician attitudes toward pharmaceutical representatives. … The rate of drug prescriptions by physicians increases substantially after they see sales representatives, attend company-supported symposia, or accept samples.”
Indeed, studies have shown that the drug company influences on doctors often lead to irrational decisions and have a negative impact on the treatment of patients.1 I am not surprised by these findings. Decades of research have allowed marketers to learn how to influence anyone without his/her knowing it. Doctors are not immune. Moreover, drug companies are subtle. They not only provide gifts and dinners and seminars, but also leave behind carefully select studies that support the use of their drugs. The overarching goal is to control the information that doctors receive about medications.
Drug companies write the package inserts of all drugs, carefully including the information they choose and omitting information they want to avoid.2Drug companies underwrite a large percentage of continuing education courses for doctors. In doing so, they make sure that the speakers represent the company view. Drug companies design studies that are meant to produce favorable results and then publish the studies in medical journals. Studies with unfavorable results are not published. Drug reps typically bring stacks of studies, all favorable, which impress doctors, who no longer have the time or motivation to search the medical literature themselves. Drug reps do not include independent studies with less favorable conclusions. Many doctors never see these.
Will True Reform Take Place?
The proposals of Brennen et al. make sense and are long overdue. But will they work? Will they even be implemented? Many calls for reform have been issued previously. Some reforms have been implemented, but they have been largely ineffective. So far, medical institutions have balked at implementing strong, enforceable rules. Doctors are independent sorts, and many still insist that they are entitled to receive gifts and other benefits if drug companies want to provide them. Academic institutions do not want to have to police their medical faculty members.
Worse, many academic institutions have become dependent on drug company money and have long become inured to drug company influences. This was best described in 2000 by Dr. Marcia Angell, then the editor-in-chief of the New England Journal of Medicine. Her astonishing article (“Is Academic Medicine for Sale?”) described a situation that has not improved:
Academic medical institutions are themselves growing increasingly beholden to industry…. Some academic institutions have entered into partnerships with drug companies to set up research centers and teaching programs in which students and faculty members essentially carry out industry research…. When the boundaries between industry and academic medicine become as blurred as they now are, the business goals of industry influence the mission of the medical schools in multiple ways….3.
With so many high-ranking doctors having financial ties to the drug industry, are there enough doctors who are independent financially or at least in conscience to consider reining in this compromised system? Maybe not. Drug companies are smart. They long ago realized that if they hired as consultants all of the top experts in every medical field, they could sway the entire medical profession with their slant. The result is that today, few independent experts remain. For example, when the FDA convenes medical advisory committees to discuss problems involving medications, it is usually impossible for the FDA to find enough independent experts to outnumber or even equal the experts with drug company ties. At some meetings, 90% of the experts have financial arrangements with drug companies.4
So the main question is not whether the proposals of Brennen et al. make sense. They do. Absolutely. The question is whether there is enough objectivity, independence, and will in the medical community to follow through. Or has the deliberate strategy of the drug industry to influence medical science and medical experts and medical institutions as much as possible already proceeded beyond control? This brings us to a question raised by another top medical journal, Lancet: “Just How Tainted Has Medicine Become?” I will discuss this article soon in Part 2 of “The Medical Profession and the Culture of Corruption.”
Finally, there is an irony in the publication of the Brennan article at this time when corruption in Washington has led to the resignation of the majority leader of the House of Representatives, and scores of politicians are trembling in anticipation of revelations by indicted lobbyist Jack Abramoff. Washington will enact reform, but will it be serious or window dressing? The same question applies to the medical profession and its conflicts of interest with the drug industry.
Newspaper Stories Monitoring Drug Industry’s
Attempt to Control Nutrition
Jun 24, 1944
Vitamin Tablets Are Ruled Drugs And General Sale in State Curbed; STATE CURBS SALE OF VITAMIN PILLS |
| Special to THE NEW YORK TIMES.. New York Times (1857-Current file). New York, N.Y.: Jun 24, 1944. pg. 1, 2 pgs |
| Abstract (Document Summary) |
| ALBANY, June 23 — Attorney General Nathaniel L. Goldstein ruled today that vitamins, when listed in the official United States Pharmacopoeia or national formulary, were drugs and could be sold at retail only by registered pharmacists, drug stores and registered stores. |
| Comment: Therefore, for the first time, the medical authorities, had found a way to eliminate competition in the sale of vitamins, by simply having vitamins declared a drug. This would also give more control to restricting the amounts of vitamins and thereby ensuring the proliferation both of disease and their drug profits. |
Jun 25, 1944
VITAMIN SALES BAN MAY LAND IN COURT; Grocers Studying Decision of Attorney General, Hint at Possible Legal Test |
| New York Times (1857-Current file). New York, N.Y.: Jun 25, 1944. pg. 32, 1 pgs |
| Abstract (Document Summary) |
| A court test of State Attorney General Nathaniel L. Goldstein’s ruling that concentrated vitamins may be sold only by pharmacists or in stores registered by the State Board of Pharmacy appeared likely yesterday as grocers protested that the ruling would give drug stores a virtual monopoly in this State on a business that amounts throughout the nation to $250,000,000 a year |
| Comment: One should note that $250.000,000 in 1944 is equal to $5,817,000,000 in 2005 dollars, giving the drug industry financial cause to suppress vitamins that were definitely competing with their drug sales. |
Dec 14, 1972
Drug Agency Acts to Restrict Use of Diet Pills and Vitamins; DRUG AGENCY ACTS FOR CURB ON PILLS |
| By RICHARD D. LYONS Special to The New York Times. New York Times (1857-Current file). New York, N.Y.: Dec 14, 1972. pg. 97, 2 pgs |
| Abstract (Document Summary) |
| WASHINGTON, Dec. 13 — The Food and Drug Administration announced moves today to restrict unnecessary and potentially harmful use of diet pills and vitamins. |
| Comment: The FDA had no proof of the harm of vitamins, so they resort to taking action due to “potentially harmful use”. |
Sep 25, 1973
Superdoses of Vitamins To Be Limited Monday |
| New York Times (1857-Current file). New York, N.Y.: Sep 25, 1973. pg. 12, 1 pgs |
| Abstract (Document Summary) |
| Barring last- minute court intervention, this is the last week that Americans will be able to buy high-concentration doses of vitamin A or vitamin D without a prescription. |
| Comment: The question to be asked is that if high doses of vitamin-A or D were harmful (note hundreds of scientific studies had proven this not to be true) then why was the drug industry allowed to sell them by subscriptions. Logic says that if it were toxic that buying it from a doctor would definitely cause as much harm as buying it from a store. |
Sep 19, 1974
F.D.A. CHIEF BACKS RULES FOR VITAMINS |
| New York Times (1857-Current file). New York, N.Y.: Sep 19, 1974. pg. 36, 1 pgs |
| Abstract (Document Summary) |
| Congress should keep its hands off the Food and Drug Administration’s plans to regulate the vitamin industry, Alexander M. Schmidt, the Commissioner of Food and Drugs, said today. |
| Comment: Of course the FDA does not want Congress who represent the people to have a say as the people have already spoken against the FDA plans. |
Sep 29, 1974
Votes in Congress; House Senate Last Week’s Tally for Metropolitan Area |
| New York Times (1857-Current file). New York, N.Y.: Sep 29, 1974. pg. 57, 1 pgs |
| Abstract (Document Summary) |
| 1. Vote on amendment providing that Food and Drug Administration regulate vitamins as foods and not as drugs, which passed, 81 to 10, Sept. 24. 2. Vote on Health Professions Educational Assistance Act, which passed, 81 to 7, Sept. 24. |
| Comment: It therefore appears that the FDA did not get its way by claiming that vitamins were drugs. They were over-ruled by the people. |
May 28, 1975
F.D.A. EASES RULES ON SOME VITAMINS; Agency Changes ’73 Decision to Control Them as Drugs A and D Still Curbed |
| By DAVID BURNHAM Special to The New York Times. New York Times (1857-Current file). New York, N.Y.: May 28, 1975. pg. 85, 2 pgs |
| Abstract (Document Summary) |
| WASHINGTON, May 27 The Food and Drug Administration backed away today from its decision of 1973 that super potent vitamins and minerals should be regulated as drugs. |
| Comment: This is ironic as the drug industry made the “super potent” vitamin-D into best selling drugs, even though they were basically the food supplement called vitamins. |
Apr 14, 1976
Congress Blocks Efforts by F.D.A. To Curb Vitamins |
| New York Times (1857-Current file). New York, N.Y.: Apr 14, 1976. pg. 16, 1 pgs |
| Abstract (Document Summary) |
| Congress has passed a bill blocking the Food and Drug Administration’s attempts to regulate the sale of high-potency vitamin and mineral pills. |
| Comment: Once again the peoples representatives speak out against the puppets of the drug industry, the FDA. |
Mar 16, 1978
F.D.A. Revokes Rules to Restrict Large Doses of Vitamins A and D |
| New York Times (1857-Current file). New York, N.Y.: Mar 16, 1978. pg. B14, 1 pgs |
| Abstract (Document Summary) |
| WASHINGTON, March 15 (UPI)–The Food and Drug Administration has revoked rules first proposed more than five years ago in an attempt to make large doses of Vitamins A and D available only with a doctor’s prescription. |
| Comment: The FDA was beaten but not down as it would continue its propaganda campaign against vitamins. (see Mar 12, 1979 report) |
Mar 12, 1979
F.D.A Panel Urges Curb On Some Vitamin Sales |
| New York Times (1857-Current file). New York, N.Y.: Mar 12, 1979. pg. B9, 1 pgs |
| Abstract (Document Summary) |
| WASHINGTON, March 11 (AP)–A Government advisory panel recommended today that curbs be imposed on sales of some vitamins and minerals as nonprescription drugs and that labels mentioning “super potency,” “natural” or “stress” be banned. |
| Comment: Once again the FDA is trying to impose the will of the drug industry on the people. |
Dec 12, 1990
Doctors Given Millions by Drug Companies |
| By WARREN E. LEARY Special to The New York Times. New York Times (1857-Current file). New York, N.Y.: Dec 12, 1990. pg. B13, 1 pgs |
| Abstract (Document Summary) |
| WASHINGTON, Dec. 11 — A Senate survey made public today said drug companies were spending more than $165 million a year on gifts, trips and payments for doctors in hopes of influencing their prescription decisions. |
| Comment: none of these doctors will prescribe life sustaining vitamins to their patients. |
Aug 9, 1992
F.D.A. Steps Up Effort to Control Vitamin Claims; F.D.A. Acts to Control Vitamin Claims
|
| By LENA WILLIAMS. New York Times (1857-Current file). New York, N.Y.: Aug 9, 1992. pg. 1, 2 pgs |
| Abstract (Document Summary) |
| In Texas, state health inspectors raided health food stores across the state in May and, as startled customers and bystanders watched in amazement, removed hundreds of products, including vitamin C, aloe vera products and herbal teas. |
| Comment: The FDA has declared war on the American people. |
Sep 8, 1992
Yes, F.D.A. Wants to Block Sale of Vitamins |
| BERNARD RIMLAND. New York Times (1857-Current file). New York, N.Y.: Sep 8, 1992. pg. A18, 1 pgs |
| Abstract (Document Summary) |
| I am one of the millions of Americans outraged by the Food and Drug Administration’s unwarranted efforts to keep me from purchasing the nutritional supplements I desire (front page, Aug. 9). My outrage is in no way mitigated by the unconvincing protestations of innocence and good intentions of David A. Kessler, the F.D.A. Commissioner, in your Aug. 16 “correction” article. |
| Comment: Apparently the FDA will never give up its war on vitamins. What the FDA needs is a nutrient savvy leader. |
Jun 15, 1993
F.D.A. Is Again Proposing to Regulate Vitamins and Supplements |
| By MARIAN BURROS Special to The New York Times. New York Times (1857-Current file). New York, N.Y.: Jun 15, 1993. pg. A25, 1 pgs |
| Abstract (Document Summary) |
| WASHINGTON, June 14 — The Food and Drug Administration will renew its efforts to control vitamins and other dietary supplements on Tuesday, when it announces plans to regulate health claims. The agency will also seek advice on how to assure the safety of the products in the $4 billion market. |
| Comment: The FDA’s efforts to destroy the vitamin market will never end.
|
|







 Did you know that experts with drug company affiliations fill many important advisory positions at the Food and Drug Administration (FDA)? An investigation by ‘USA Today’ found that more than half of the experts on Food and Drug Administration (FDA) advisory committees have financial relationships with the drug companies which will be either hurt or helped by their decisions (5).
Did you know that experts with drug company affiliations fill many important advisory positions at the Food and Drug Administration (FDA)? An investigation by ‘USA Today’ found that more than half of the experts on Food and Drug Administration (FDA) advisory committees have financial relationships with the drug companies which will be either hurt or helped by their decisions (5). Collectively, the 10 drug companies in the Fortune 500 topped all 3 of the Fortune magazine’s measures of company profitability for 2001, according to the magazine’s annual analysis of America’s most important companies.
Collectively, the 10 drug companies in the Fortune 500 topped all 3 of the Fortune magazine’s measures of company profitability for 2001, according to the magazine’s annual analysis of America’s most important companies. Some of these withdrawn drugs, such as ‘Redux’, ‘Seldane’, ‘Propulsid’, ‘Rezulin’ , were prescribed MILLIONS OF TIMES. According to Dr. Alastair J.J. Wood, Assistant Vice Chancellor for Research at the Vanderbilt University Medical Center (1), a staggering 19.8 million patients (almost 10% of the United States population) were estimated to have been exposed to just 5 of the 10 drugs withdrawn in the past 10 years. (see below for the list)
Some of these withdrawn drugs, such as ‘Redux’, ‘Seldane’, ‘Propulsid’, ‘Rezulin’ , were prescribed MILLIONS OF TIMES. According to Dr. Alastair J.J. Wood, Assistant Vice Chancellor for Research at the Vanderbilt University Medical Center (1), a staggering 19.8 million patients (almost 10% of the United States population) were estimated to have been exposed to just 5 of the 10 drugs withdrawn in the past 10 years. (see below for the list)